Buzz word
Graphene is a single atomic layer of graphite. The term “graphene” was precisely defined since 1986 recommended by Boehm: “the ending -ene is used for fused polycyclic aromatic hydrocarbons (融合多环芳烃). A single carbon layer of the graphitic structure would be the final member of infinite size of this series. The term graphene layer should be used for such a single carbon layer.”[1] In 1997, IUPAC formalized these recommendations by incorporating them into their Compendium of Chemical Technology, which states: “….. The term graphene should be used only when the reactions, structural relations or other properties of individual layers are discussed.”
History of Graphene
1947 Wallace, developed the linear E(k) dispersion for the electronic structure of graphene around the K point of the Brillouin zone.
1962 Monolayer and few-layer graphene was quietly synthesized by Boehm using chemical thermal reduction of graphite oxide, who became better known starting in the 1970s for his work on graphite intercalation compounds (GICs).
1968 Morgan and Somorjai obtain LEED patterns produced by small-molecule adsorption onto Pt(100).
1969 May interprets the data collected by Morgan and Somorjai as the presence of a monolayer of graphene on the Pt surface.
1970 Blakely and co-workers prepare monolayer graphite by segregating carbon on the surface of Ni(100).
1975 van Bommel and co-workers make graphene by subliming silicon from silicon carbide.
1986 Boehm and co-workers recommend that the term “graphene”.
1997 IUPAC formalizes the definition of graphene.
1999 Ruoff and co-workers micromechanically exfoliate graphite into thin lamellae comprised of multiple layers of graphene.
2001 Walt de Heer, reported on carbon conferences on fabrication of a planar graphene layer by heating SiC to 1300C and on the physical properties of these layers.
In the same year, Enoki and co-workers in Japan were preparing monolayer and few-layer graphene ribbons to examine their structural and magnetic properties, mostly, edge properties.
Shortly thereafter, Philip Kim and collaborators developed a mechanical exfoliation process for producing few-layer graphene samples.
2004 Geim and Novoselov used a simple mechanical exfoliation method for preparing monolayer and few-layer graphene and repeatedly transferring their graphene samples to the desired surface. They made many breakthrough physics discoveries they made in a short period of time.
2010 Nobel Prize of Physics is awarded to Geim and Novoselov for their research on graphene.
Future The significance of carbon nanostructures have been demonstrated by awarding many Nobel Prizes to the discovery of new carbon nanostructures. Every entry of new carbon nanostructure would have great impact on scientific literatures. No wonder why the graphene is a hot researching area now. Specifically in nanoelectronics, we can expect the coming of era of carbon-based nanoelectronics in near future.

Fig 1. History of publications on carbon-based materials [2].
Distinguished structures and properties
Carbon, has four valence electrons in the ground state, two in the 2s subshell and two in the 2p subshell. By promoting one of its 2s electrons into its empty 2p orbital, it forms bonds with other carbon atoms via sp hybrid orbitals. There are three kinds of sp hybrid orbitals, i.e., sp, sp2 and sp3 hybrid orbitals. 2p orbitals hybridize with one s orbital to form three sp2 orbitals, which subsequently form the so-called σ bonds with the three nearest-neighbor carbon atoms in the honeycomb lattice. The σ bonds do not contribute to electrical conduction. An ideal graphene is a monatomic layer of carbon atoms arranged on a honeycomb lattice, as shown in Fig 2, which is a perfect two-dimensional (2D) material.
The unit cell spanned by the following two lattice vectors:

Here a=0.142 nm, is the carbon bond length. The corresponding reciprocal lattice vectors are given by
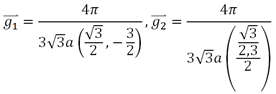
Which also form a honeycomb lattice. Of particular interest inside the first BZ are two points K and K`. The A and B lattices decouple, forming the so-called Dirac point.

Fig 2. Unit cell lattice structure and first BZ of graphene
Among all carbon allotropes, graphene stands out because of its quasirelativistic low-energy excitations near the two unequivalent K point at the corners of the first Brillouin zone; the quasiparticles are chiral and massless Dirac fermions with the electrons and holes degenerated at the Dirac points. This gives rise to a number of peculiar physical properties include unconventional integer quantum Hall effect (IQHE), Klein tunneling, valley polarization, universal minimum conductivity, week localization (WAL), ultrahigh mobility, specular Andreev reflection at the graphene-superconductor interface, etc. Graphene is an interesting mix of semiconductor, metal and soft matter.
| Table 1. Physical properties of single-layer graphene at room temperature | ||
| C-C bond length in SLG, nm | 0.142 | Thinnest material in the world |
| Layer distance, nm | 0.34 | Loose stack, only van der Waals force |
| Specific Surface, m2/g | ≈2630 | Gas and energy storage, ultracapacitors |
| Electron mobility, cm2/(V∙s) | ≈4.4*104[3] | Channel material for RF, transistor post-CMOS |
| Young’s modulus, TPa | ≈1 | NEMS, pressure sensors and resonators |
| Transparency | ≈90% | Solar cell, optoelectronics |
| Thermal conductivity, W/(m∙K) | ≈5.1*103 | |
| Magnetotransport | Spintronics | |
Other potential applications are Single molecule gas detection, DNA detection and sequencing.
Reference
[1] D.R. Dreyer, R.S. Ruoff, and C.W. Bielawski, “From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future,” Angewandte Chemie International Edition, Nov. 2010, p. n/a-n/a.
[2] P. Avouris, “Graphene: Electronic and Photonic Properties and Devices,” Nano Letters, Sep. 2010, p. 100929092847080.
[3] R.S. Shishir and D.K. Ferry, “Intrinsic mobility in graphene,” Journal of Physics: Condensed Matter, vol. 21, Jun. 2009, p. 232204.
PS: A graph from WellHome introduces graphene in a interesting way
Supplement Resources for who wants to step into graphene area:
Updated on 2011-11-02
Excellent review articles of graphene
- Geim, a K. & Novoselov, K.S. The rise of graphene. Nature materials 6, 183-91 (2007).
- Geim, a K. Graphene: status and prospects. Science (New York, N.Y.) 324, 1530-4 (2009).
- Castro Neto, a, Guinea, F., Peres, N., Novoselov, K. & Geim, A. The electronic properties of graphene. Reviews of Modern Physics 81, 109-162 (2009).
- Das Sarma, S., Adam, S., Hwang, E. & Rossi, E. Electronic transport in two-dimensional graphene. Reviews of Modern Physics 83, 407-470 (2011).
- Choi, W., Lahiri, I., Seelaboyina, R. & Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Critical Reviews in Solid State and Materials Sciences 35, 52-71 (2010).
- Avouris, P. Graphene: Electronic and Photonic Properties and Devices. Nano Letters 100929092847080 (2010).doi:10.1021/nl102824h
- Schwierz, F. Graphene transistors. Nature Nanotechnology 5, 487-496 (2010).
- Singh, V. et al. Graphene Based Materials: Past, Present and Future. Progress in Materials Science (2011).doi:10.1016/j.pmatsci.2011.03.003
- Bonaccorso, F., Sun, Z., Hasan, T. & Ferrari, a C. Graphene photonics and optoelectronics. Nature Photonics 4, 611-622 (2010).
- Cooper, D.R. et al. Experimental review of graphene. arXiv (2011).
Awesome online course
- Joerg Appenzeller; Supriyo Datta; Mark Lundstrom (2009), “Colloquium on Graphene Physics and Devices,” http://nanohub.org/resources/7180.
- Supriyo Datta (2004), “ECE 453 Lecture 21: Graphene Bandstructure,” http://nanohub.org/resources/686.


不理解K和K’,lattice有什么特性呢
好好学习,靠你了